National Pharmaceutical Control Bureau
Year no of ct title no of sites 2000 2a prospective randomized.
National pharmaceutical control bureau. Npra formerly known as the national pharmaceutical control bureau bpfk operates at jalan universiti petaling jaya since its inception in october 1978. Npra is responsible for ensuring that the malaysian consumers obtain quality effective and safe remedies. Ar 2293 non oecd member full adherence to the mutual acceptance data mad system national pharmaceutical control bureau npcb ministry of health malaysia 1st malaysia japan symposium.
As npra s mission is to safeguard the nation s health npra has kept up to date with current situations and provide guidance for during cmco and for this pandemic period. National pharmaceutical control bureau ministry of health malaysia pharmazie jobs anzeigen folgen alle 80 mitarbeiter anzeigen dieses unternehmen melden mitarbeiter von national pharmaceutical control. This unit is responsible for the evaluation and registration of biological products which was previously undertaken by the poisons unit of the division.
National pharmaceutical control bureau ministry of health malaysia in moses lake wa jobs personen e learning verwerfen verwerfen. National pharmaceutical control bureau npcb drug regulatory agency of malaysia. National pharmaceutical control bureau npcb who collaborating centre for regulatory control of pharmaceuticals member of pharmaceutical inspection cooperation scheme ms iso 9001 2008 certified cert no.
Formerly known as national pharmaceutical control laboratory. National pharmaceutical control bureau the biotechnology unit which is a unit under the product evaluation and safety division of the national pharmaceutical control bureau npcb was formed in may 2002. The national pharmaceutical control bureau npcb malaysia formerly known as the national pharmaceutical control laboratory was set up in october 1978 under the quality control activity of pharmacy and supply programme.
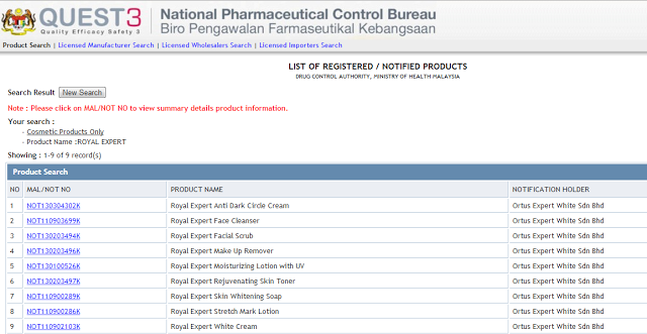
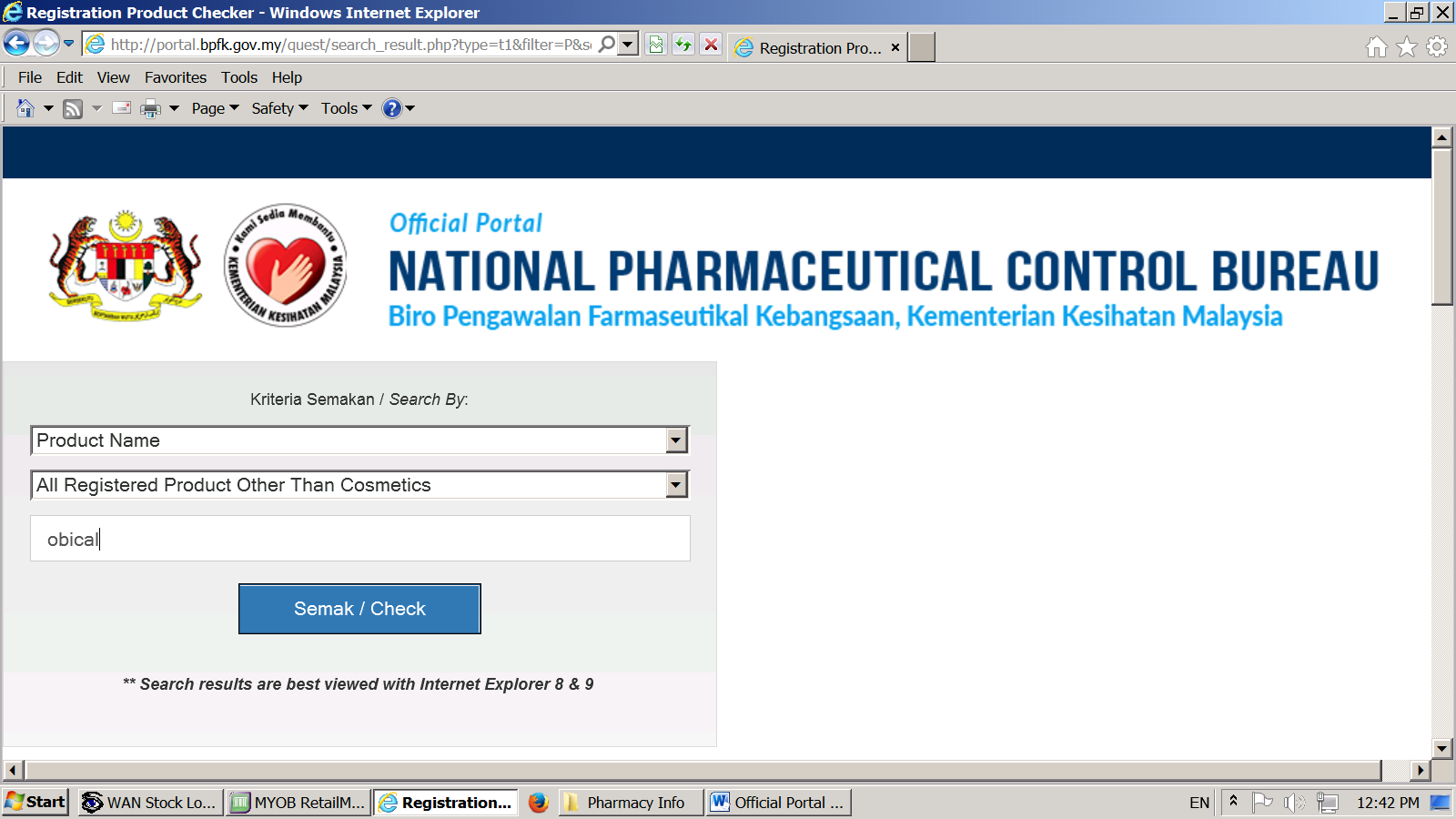
1 drug related clinical trials for registered products which do not require clinical trial import license is not controlled by the drug control authority. The national pharmaceutical control bureau biro pengawalan farmaseutikal kebangsaan bpfk shall not be liable for any loss or damage caused by the usage of any information obtained from this website. 1 statistics are based on the number of applications received by national pharmaceutical control bureau for the clinical trial import license for unregistered products.
Also known as biro pengawalan farmaseutikal kebangsaan in malay term. For a complete organizational.